Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Arakawa, Masahiko*; Yasui, Minami
Physics and Chemistry of Ice 2010, p.339 - 344, 2011/05
We conducted impact experiments of water ice and measured a post shock temperature of impact crater using an infrared video camera. The surface temperature in a crater showed a peak temperature just after the impact then it cooled down gradually with time. The decay time of maximum temperature might depend on a scale of heated volume in a crater, so we estimated the ratio of thermal energy to projectile kinetic energy. As a result, we found that up to 10% of the projectile kinetic energy was partitioned into the post shock heating of the impact crater.
Shimaki, Yuri*; Arakawa, Masahiko*; Yasui, Minami
Physics and Chemistry of Ice 2010, p.379 - 386, 2011/05
Impact disruption of sintered snowballs was studied to clarify the effect of sintering on impact strength and fragment velocity. Ice and snow projectiles were impacted on sintered snowballs with a porosity of 40%. The sintering duration of snow was changed from 1 hour to 1 month, and the effect of sintering on impact strength and static strength were examined. As a result, it was found that both impact strength and static strength of snow have a power law relationship to a sintering duration with a power law index of about 0.2.
Yasui, Minami; Arakawa, Masahiko*
Physics and Chemistry of Ice 2010, p.387 - 395, 2011/05
We did deformation experiments on ice-silica mixtures to study their flow laws. We found that the mixture became softer as porosity or silica mass content increased. A power law index  and an activation energy
and an activation energy 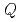 were found to depend only on silica mass content.
were found to depend only on silica mass content.
Fukazawa, Hiroshi; Arakawa, Masashi*; Kagi, Hiroyuki; Yamauchi, Hiroki; Chakoumakos, B. C.*; Fernandez-Baca, J. A.*
Physics and Chemistry of Ice 2010, p.421 - 428, 2011/03
Whether or not ice in the universe exists as ferroelectric is a question that has attracted interest in astrophysics and physical chemistry, because long-range electrostatic forces, caused by the ferroelectricity, might be an important factor for materials evolution and hydrogen bonding. From neutron diffraction and scattering measurements, we have studied ice with impurities, such as potassium, sodium and lithium, that acted as a catalyst. Time-resolved diffraction experiments show that ferroelectric ice XI with hydrogen-ordered arrangement nucleates and grows with time for about 5 days. We found that the doped ice that has once been converted to ice XI is a stronger ferroelectric ice than that has never been converted. We also show the existence of the ferroelectric ice under high-pressure and its formation from compressed amorphous ice.
Arakawa, Masashi*; Kagi, Hiroyuki; Fernandez-Baca, J. A.*; Chakoumakos, B. C.*; Fukazawa, Hiroshi
Physics and Chemistry of Ice 2010, p.329 - 338, 2011/03
We measured neutron diffraction profiles of KOD, NaOD, LiOD, Ca(OD) , and ND
, and ND -doped ices. Ice XI, which is a hydrogen-ordered phase of normal ice (ice Ih), was observed in the KOD and NaOD-doped ices although Ca(OD)
-doped ices. Ice XI, which is a hydrogen-ordered phase of normal ice (ice Ih), was observed in the KOD and NaOD-doped ices although Ca(OD) and ND
and ND -doped ice did not transformed to ice XI. The mass fraction of ice XI to that of the doped ice (
-doped ice did not transformed to ice XI. The mass fraction of ice XI to that of the doped ice ( ) was estimated using Rietveld analysis for each doped ice. The
) was estimated using Rietveld analysis for each doped ice. The  value of the doped ice, which had once experienced being ice XI, was larger than that of the doped ice, which had never experienced being ice XI. The large
value of the doped ice, which had once experienced being ice XI, was larger than that of the doped ice, which had never experienced being ice XI. The large  value of the doped ice indicates that small hydrogen-ordered domains remained above the transition temperature between ice XI and Ih. Our results suggest that large amounts of ice on icy bodies in our solar system can transform to ice XI. In this paper, we discussed the existence of the small hydrogen-ordered domains in space and the evolution of icy grain.
value of the doped ice indicates that small hydrogen-ordered domains remained above the transition temperature between ice XI and Ih. Our results suggest that large amounts of ice on icy bodies in our solar system can transform to ice XI. In this paper, we discussed the existence of the small hydrogen-ordered domains in space and the evolution of icy grain.
Kikuchi, Tatsuya; Matsumoto, Masakazu*; Yamamuro, Osamu*
no journal, ,
We are studying the formation mechanism of gas hydrates currently attracting much attention in the research field of clathrate hydrates. The largest difficulty for this study is that guest gas molecules hardly dissolve into water under ambient pressure. In order to overcome this difficulty, we prepared aqueous solutions with high solubility (2% at maximum) of guest gases by applying gas high-pressure to water. The quasi-elastic neutron scatterings (QENS) of these samples have been measured to investigate the dynamics of water molecules affected by the guest molecules. The measurements were carried out on AGNES spectrometer installed at JRR-3 (JAEA, Tokai) and maintained by ISSP, University of Tokyo. The guest molecules taken in this study were Ar, Xe, N , and CO
, and CO which have simple molecular structures. The pressure and temperature ranges were 0-100 MPa and 263-363 K, respectively. We analyzed the QENS spectra
which have simple molecular structures. The pressure and temperature ranges were 0-100 MPa and 263-363 K, respectively. We analyzed the QENS spectra 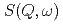 based on the jump diffusion model. The diffusion coefficient
based on the jump diffusion model. The diffusion coefficient  is smaller than
is smaller than  of pure water especially below the hydrate-formation temperature
of pure water especially below the hydrate-formation temperature 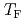 and for the guest molecules with high solubility. These experimental results were reproduced well by MD simulations. It was also found that the gas molecules get closer to each other and the diffusion of water molecules near gas molecules is suppressed, resulting in the smaller diffusion coefficient below
and for the guest molecules with high solubility. These experimental results were reproduced well by MD simulations. It was also found that the gas molecules get closer to each other and the diffusion of water molecules near gas molecules is suppressed, resulting in the smaller diffusion coefficient below 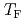 .
.